Soft Ionization Mass Spectrometry
Noncovalent interactions are at the heart of biological chemistry. Indeed, these interactions are involved in every single biological process, from the intramolecular folding of proteins and nucleic acids to the molecular recognition of their targets.
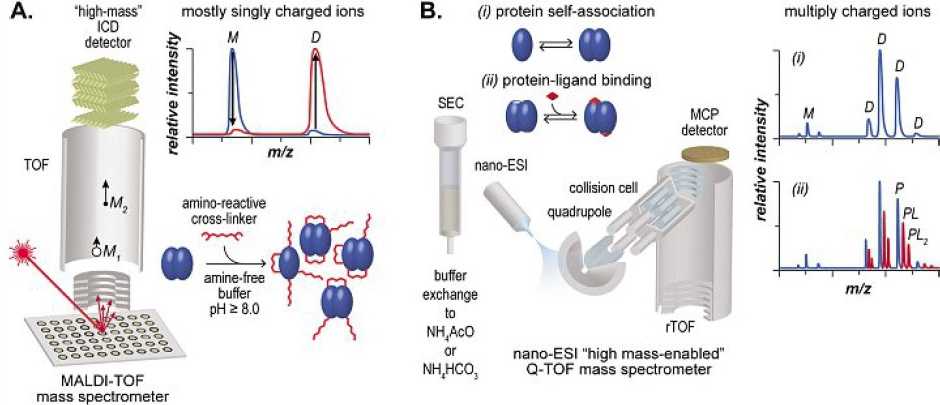
With conventional biophysical techniques, it is challenging to study mixtures of complexes with either different stoichiometries or structures. Using mass spectrometry (MS), it has become possible to detect and quantify complexes made of multiple stoichiometries simultaneously. We use soft ionization methods, namely matrix-assisted laser desorption ionization (MALDI) and electrospray ionization (ESI) for the ionization of large (>10 kDa) biomolecules under native conditions. This has been achieved either by chemical cross-linking followed by high-mass MALDI MS analysis (Figure 1A) or by “native electrospray ionization” (Figure 1B). Native MS, as a prominent analytical technique, has found increasing interest in various biochemical/biophysical fields allowing us to investigate noncovalent complexes of small molecules, oligonucleotides, or proteins.
One focus of our group is the study of large noncovalent protein complexes using a special high-mass detector. Noncovalent interactions of complexes are first stabilized with “chemical glues” (cross-linkers) and detected thanks to a high-mass sensitive ion conversion dynode technology implemented in our MALDI MS instrument.
Using ESI mass spectrometry, it is possible to maintain noncovalent complexes intact and unambiguously access the ensemble of stoichiometries present in the solution under the conditions used. Affinity constants and kinetics parameters can be obtained this way. Further structural characterization of the complexes in the gas phase can be obtained using ion mobility spectrometry (IMS) coupled with MS.
Temperature-controlled ESI MS
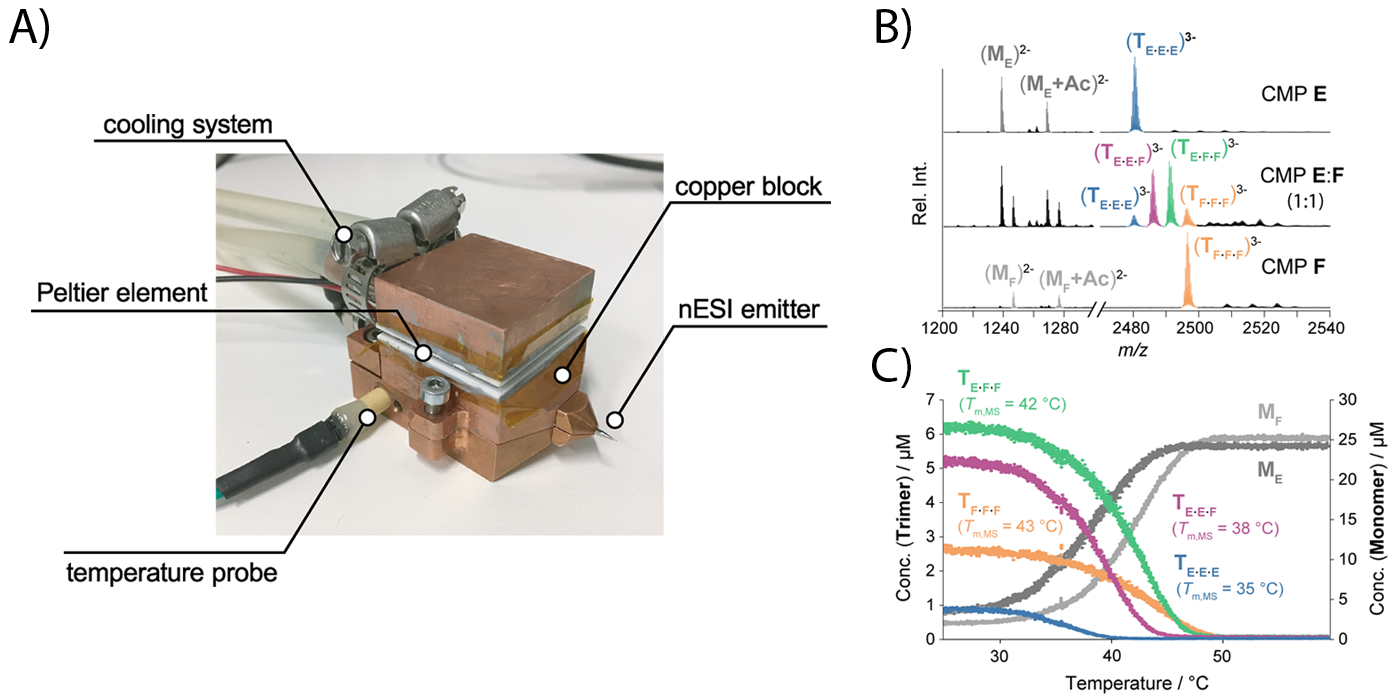
The strength of native MS lies in its ability to detect and quantify all stoichiometries coexisting in a mixture unambiguously. However, in a commercial instrument, ESI MS experiments are typically only conducted at room temperature. This limits its utility as a biophysical technique because the determination of the enthalpies and entropies requires temperature-dependent experiments. To address this shortcoming, we built a nanoESI source with which the temperature of the sprayed solution can be varied from 15 to 95 °C. This is achieved by embedding the nanospray emitter inside a temperature-controlled copper block. A clear advantage of temperature-controlled nanoESI MS over conventional techniques is the possibility to determine complex binding equilibria in a more straightforward manner since each species existing in solution appears at a different mass-to-charge ratio in a mass spectrum.
Cyclic Ion Mobility ESI-MS
The past decade has seen an increase in commercially available instruments which can analyze noncovalent complexes by ion mobility. This technique allows researchers to gain a more detailed understanding of a biomolecules structure. Our lab has a Select Series Cyclic IMS mass spectrometer. This instrument has several advantages over other commercially available ion mobility platforms, such as the ability to pass ions through the ion mobility cell multiple times for greater separation and the isolation analytes based on their geometry (drift time). By applying this technique, in conjunction with other methods (such as TC-nESI), it is possible to gain access to structural information which is not readily accessible to other techniques, such as temperature induced conformational changes.
High-mass MALDI mass spectrometry
Investigation of Membrane Protein Interactions via high-mass MALDI-TOF and chemical crosslinking (XL). In previous studies in our lab, chemical cross-linking combined with high-mass MALDI MS has been successfully applied in numerous systems, for example, antigen-antibody interactions, hemoglobin complexes, and hormone receptor complexes. Applying this method to intact membrane complexes is still challenging due to difficulties to solubilize them. Besides, the absence or low abundance of polar amino acid side chains render membranes proteins difficult to ionize.
Different conformational states of G protein-coupled receptors (GPCRs) and their partners are precisely and cleanly reflected by intensity changes of various m/z features in the MALDI data, helping to uncover binding to GPCRs in the active and inactive states. Our approach provides a fast, convenient, and sensitive way to explore the ligands induced assembly of GPCR complexes. We have also used this technique to investigate nanobody•ABC transporter complexes to screen for nanobodies with suitable binding specificities. The results could be used for the optimization of protein complex crystallization conditions, in cases where nanobodies are used to stabilize flexible protein regions.
Current projects:
Recently, we examined the temperature-dependent binding behavior of DNA duplexes and G-quadruplexes in complex with a series of ligands. The potential of TCnESI has also been demonstrated in the study of collagen peptide complexes. Here we investigated heterotrimeric helices that are difficult to characterize by NMR and CD spectroscopic techniques. Current projects focus on thermodynamics of multi-domain complexes and interaction between DNA secondary structures and binding proteins, as well as using ion mobility to investigate the thermal behavior of biomolecular conformers, protein aggregation and isomeric/isobaric collagen model peptides.
Collagen naturally occurs in a triple helical, fibrous structure and plays a key role in the stability of skin, connective tissue or bones as the most abundant mammalian protein. Synthetic collagen shows huge potential for use as biomaterials and in probing tumors. The formation process of heterotrimeric polyproline type II triple helices from collagen model peptides (CMPs) is one of the core research topics in the group of Prof. H. Wennemers group (ETHZ). Over the last three years, our collaboration with the Wennemers group has successfully shown the advantages of applying temperature-controlled nESI-MS, IM-MS and also cyclic IM-MS for studying CMPs. As opposed to CD spectroscopy, which is traditionally used to characterize CMPs, temperature-controlled nESI-IM-MS gives unique access to information about the sample complexity, the selectivity of helix formation, and thermodynamic parameters of each compound in a complex mixture.
Micro-heterogeneities in monoclonal antibodies and immune regulatory proteins such as their glycosylation pattern, aggregation, oxidation or deamidation have a direct impact on their efficacy and affinity to their target. the mAb mediated anti-tumor mechanism and This can often not be fully characterized by classical biophysical methods or liquid chromatography mass spectrometry. To overcome this limitation, our group is using a combination of native ESI-MS, (cyclic) IMS and different fragmentation techniques such as CID, SID and ECD to study those heterogeneities and establish novel workflows for the structural characterization of biopharmaceuticals.
Our lab is also interested in identification of antibody binding targets from complex mixtures, as well as their affinities. Recently, we started investigating the binding activity of monoclonal antibodies against multiple snake venoms to help develop better treatments for snake bites, which is classified as a neglected tropical disease that effects millions of people every year.
DELs have recently been demonstrated to be a fast and cost-effective platform for the discovery of novel small molecules. These libraries consist of thousands to millions of different small molecules, which are all labeled with a unique DNA sequence serving as a “barcode” for a later hit identification. By mixing the labeled molecules with a putative drug target (protein), binders can later be read out by PCR amplification. However, it remains unclear how binding constants are influenced by the molecule’s DNA tags, as binding of small molecules could be altered by steric or electrostatic effects of the DNA label itself. In collaboration with the group of Dr. J. Scheuermann (ETHZ), which focuses on the development of novel DECL approaches, we plan to investigate the binding properties of small molecules compared to their DNA-tagged variants. Native ESI-MS offers here a truly label-free method to characterize these systems in the gas phase.
The entry of SARS-CoV-2 viruses into cells is mediated by the binding of the spike (S) protein of SARS-CoV-2 to the angiotensin-converting enzyme 2 (ACE2) receptor on cell surfaces. Both the S protein and ACE2 are glycosylated, which could affect the protein folding and the binding of S with ACE2. Here, we utilized crosslinking MALDI-MS with high mass detection (MALDI-HMMS) for rapid profiling the effects of glycosylation on the binding of the S protein receptor binding domain (RBD) to ACE2. On the other hand, small drug molecules have the possibility to interfere with the binding of S protein to ACE2. We also used MALDI-HMMS to quantitatively evaluate the inhibition of the binding of S protein RBD to ACE2 by FDA approved small drug molecules. MALDI-HMMS based methods reveal the possible interactions and glycosylation effects on SARS-CoV-2 cell entry, and introduce a robust method for drug screening in noncovalent interaction involved virus infection.